
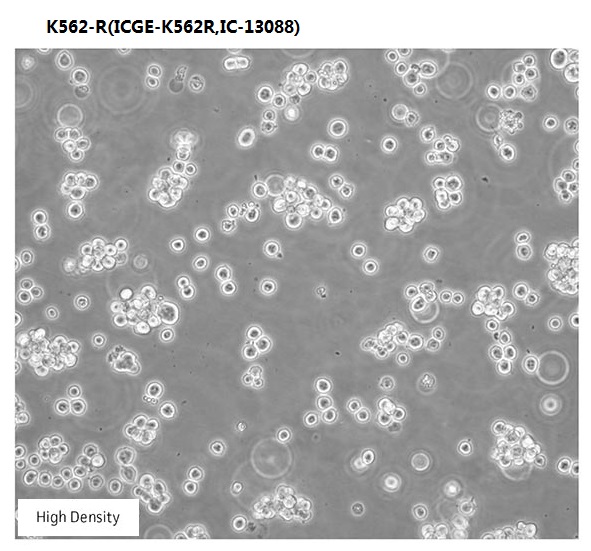
Overview
| Organism | Homo sapiens, human |
|---|---|
| Tissue | bone marrow; derived from metastatic site: pleural effusion |
| Product Format | frozen 1.0 mL |
| Morphology | hematopoietic |
| Culture Properties | suspension |
| Biosafety Level |
1
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | chronic myeloid leukemia at blast crisis |
| Age | 53 |
| Gender | female |
| Applications |
This is a clone of the parental K562 cell line which is resistant to the tyrosine kinase inhibitor imatinib mesylate, and is paired to the K562-s clone which is sensitive to imatinib. The paired K562-s and K562-r clones are useful cell line models of sensitivity and resistance to a tyrosine kinase inhibitor used in the treatment of chronic myeloid leukemia. |
| Storage Conditions | liquid nitrogen vapor phase |
Properties
|
|
|
|---|---|
| Derivation | Erythroid-Myeloid precursor cell line derived from chronic myeloid leukemia (CML) in blast crisis. |
| Clinical Data |
female 53 years |
| Oncogene | BCR-ABL of the e14a2 fusion transcript type |
| Comments |
Oncogenes: BCR-ABL of the e14a2 fusion transcript type Erythroid-myeloid precursor cell line derived from chronic myeloid leukemia (CML) in blast crisis |
Background
| Complete Growth Medium |
The complete medium for this cell line is RPMI-1640 + 10% FBS + 2 mM L-Glutamine + 1 ��M Imatinib Details for preparation of 500 ml of complete media
|
|---|---|
| Subculturing |
Cultures can be maintained by addition or replacement of fresh medium. Start cultures at 2.5 to 3.5 X 105 cells/mL and maintain between 2 X 105 and 1 X 106 cells/mL.
Medium Renewal: Add fresh medium every 2 to 3 days (depending on cell density). |
| Culture Conditions |
Atmosphere: air, 95%; carbon dioxide (CO2), 5%
Temperature: 37��C
|


