Overview
| Permits and Restrictions | |
|---|---|
| Organism | Homo sapiens, human |
| Tissue | pancreas; Derived from metastatic site: spleen |
| Product Format | frozen |
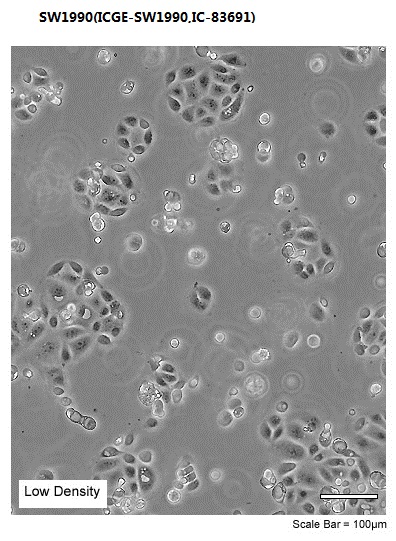
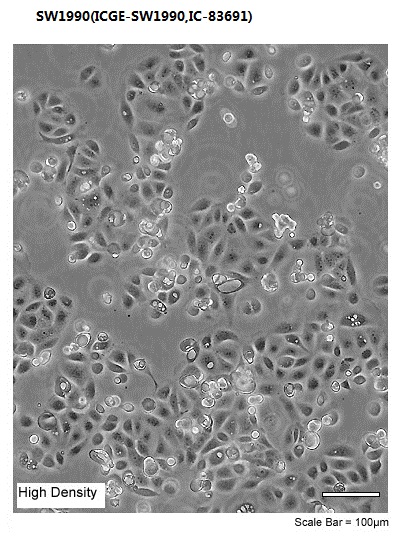
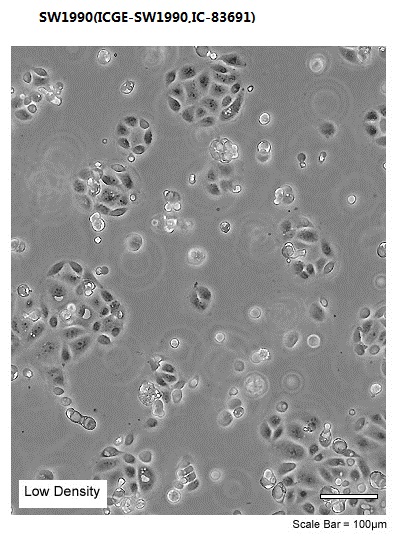
| Morphology | Epithelial |
| Culture Properties | adherent |
| Biosafety Level |
1
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | adenocarcinoma |
| Age | 56 years |
| Gender | Male |
| Ethnicity | Caucasian |
| Storage Conditions | liquid nitrogen vapor phase |
Properties
| Karyotype | near triploid; range = 67 to 75 |
|---|---|
|
|
|
| Derivation |
The SW 1990 line was established in 1978 from a spleen metastasis of a grade II pancreatic adenocarcinoma derived from the exocrine pancreas.
|
| Clinical Data |
56 years
Caucasian
male
|
| Antigen Expression |
Blood Type A; Rh +
|
| Tumorigenic | Yes |
| Effects |
Yes, forms tumors in nude mice
|
| Comments |
The cells have a reported plating efficiency of 29%.
|
Background
| Complete Growth Medium |
The base medium for this cell line is ATCC-formulated Leibovitz''''''''s L-15 Medium, Catalog No. 30-2008. To make the complete growth medium, add the following components to the base medium: fetal bovine serum to a final concentration of 10%.
(Note: The L-15 medium formulation was devised for use in a free gas exchange with atmospheric air. A CO2 and air mixture is detrimental to cells when using this medium for cultivation) |
|---|---|
| Subculturing |
Subcultivation Ratio: A subcultivation ratio of 1:2 to 1:4 is recommended
Medium Renewal: 2 to 3 times per week
|
| Cryopreservation |
Freeze medium: Complete growth medium supplemented with 5% (v/v) DMSO
Storage temperature: liquid nitrogen vapor phase
|
| Culture Conditions |
Temperature: 37��C
Atmosphere: air, 100%
|


